Reliance Agreement
SMART IRB Agreement
Supporting IRB reliance across the nation
Reliance Agreement Version 3.0 is Now Available!
As of March 17, 2025, institutions must join Version 3.0 to initiate any new SMART IRB reliance arrangements.
SMART IRB Reliance Agreement Version 3.0
May be used to facilitate IRB reliance among Participating Institutions for a wide range of studies.
Before joining, review the Agreement and optional Indemnification Addendum with institution officials and counsel.
Do not sign the sample Joinder Agreement. You will use the Reliance System to join the Agreement.
Related Documents
Questions? Connect with an Ambassador or Contact Us.
Using the Agreement
The SMART IRB Agreement:
- Enables reliance on a study-by-study basis and may be used to facilitate multiple reliances between institutions or for consortiums or other groups.
- Clearly defines roles and responsibilities
- Eliminates the need to sign reliance agreements for each study.
Once you’ve joined SMART IRB, you may use the Agreement to support collaborations among Participating Institutions*. Simply document the arrangement in the Reliance System, or via another mechanism (e.g., Template Letter of Acknowledgement).
*Note: Institutions must join SMART IRB Reliance Agreement V3.0 for any new reliance arrangements.
Prior versions of the SMART IRB Agreement (V1.0/V2.0) are now considered Legacy Agreements; while previously documented arrangements under those agreements remain valid, Legacy Agreements may not be used for any new reliance arrangements.
Which Agreement Applies?
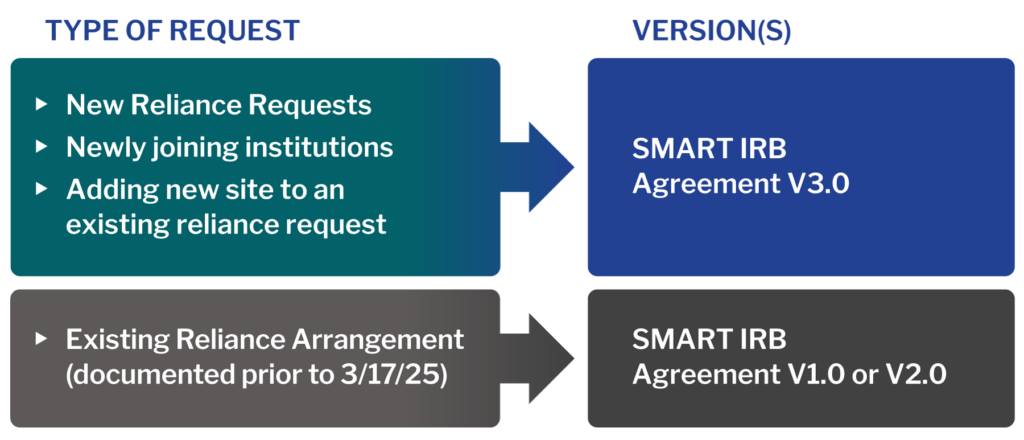
Please note that while institutions may mutually agree to transition Legacy reliance arrangements to V3.0, institutions may not transition V3.0 arrangements to the Legacy Agreements.
Indemnification
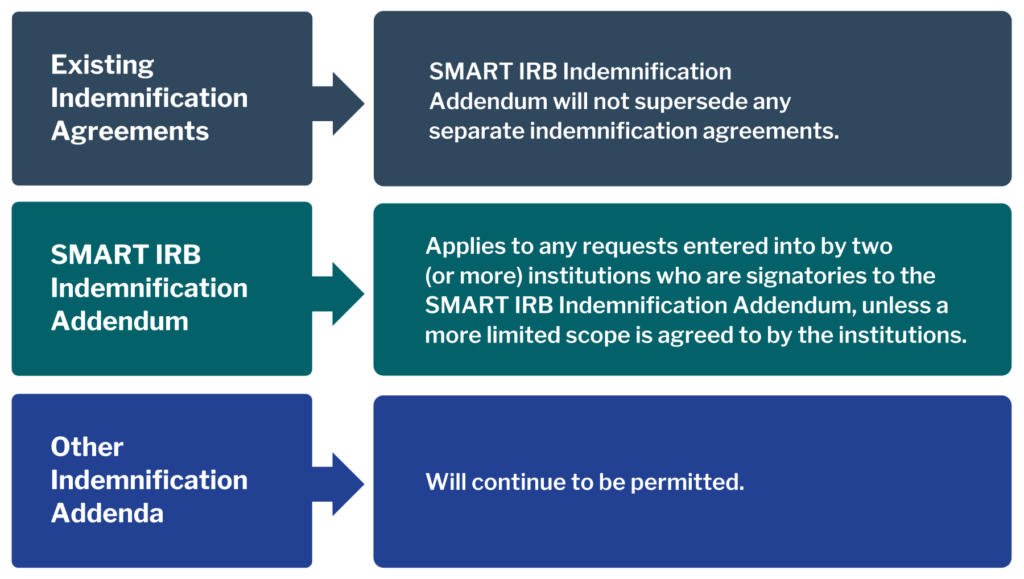
The SMART IRB Agreement does not require institutions to indemnify one another. The SMART IRB Indemnification Addendum is offered as an option for institutions that wish to sign it. It was developed to harmonize approaches and reduce the need to negotiate individual indemnification agreements.
The SMART IRB Indemnification Addendum is attached to the Agreement at Exhibit C. Institutions wishing to do so may generate and sign their joinder for the addendum through the Reliance System. Learn more.
Indemnification & The SMART IRB Agreement
Background
SMART IRB Agreement Version 1.0 was available from September 5, 2016 – September 30, 2020, when SMART IRB Agreement Version 2.0 became available. Because these versions of the Agreement were compatible, Participating Institutions were not required to sign SMART IRB Agreement Version 2.0.
Over the next years, SMART IRB leadership worked with the SMART IRB Harmonization Steering Committee, the National Center for Advancing Translational Sciences (NCATS) and other federal agency representatives, and members of the SMART IRB community to discuss possible changes to the Agreement.
The resulting SMART IRB Reliance Agreement Version 3.0 is a significant amendment, intended to:
- address feedback from current and potential Participating Institutions
- fully reflect changes to IRB review requirements in the 2018 Common Rule; and
- allow additional federal agencies to participate in the agreement.
All current Participating Institutions and stakeholders, as well as prospective signatories, were provided opportunity to comment on the draft SMART IRB Reliance Agreement Version 3.0 between November 15, 2023 and February 15, 2024. During this initial comment period, we received 250 unique comments from 54 different institutions. Several substantive changes were made in response and incorporated into a revised draft Version 3.0, which was available for a second review period from November 13 – December 13, 2024. The final SMART IRB Reliance Agreement V3.0 takes into consideration comments received, with the aim of enabling the broadest possible participation and utility.
As of March 17, 2025, any new institutions will join SMART IRB Reliance Agreement V3.0, and Participating Institutions must join V3.0 to document any new SMART IRB reliance arrangements. The prior versions of the SMART IRB Agreement (now considered Legacy Agreements) remain valid for previously documented arrangements. However, Legacy Agreements may not be used for any new reliance arrangements (including adding sites to an existing study).
Questions? Contact us.
Join SMART IRB
Join our growing IRB reliance network to streamline your reliance arrangements and facilitate collaborations across the nation. Learn More.